The research in the Sui Lab primarily focuses on developing advanced diagnostic and therapeutic techniques for biomedical applications. We synthesize small organic molecules and biocompatible and biodegradable polymers with predesigned functions, and then employ them to fabricate smart nanosystems loaded with various agents via stimuli-responsive and self-immolating linkers, serving as diagnostic and/or therapeutic techniques for the treatment of human diseases, including cancers and neurological disorders. Owing to the multidisciplinary nature of the research projects, students working in the Sui Lab will have the opportunity to learn a broad range of skills and expertise in organic synthesis, polymer chemistry, fluorescence spectroscopy, materials characterization, nanotechnology, electron microscopy, optical microscopy, biochemistry, molecular biology, cellular biology, animal studies, and biomedical engineering.
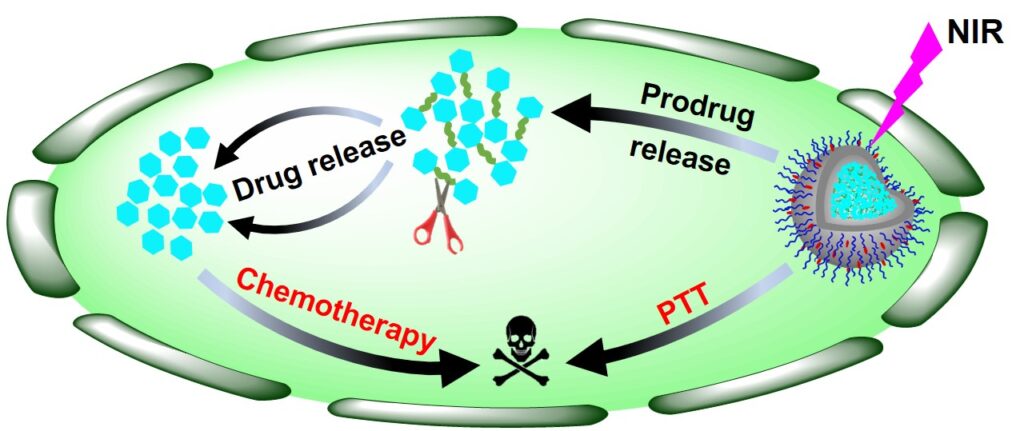
1. Theranostic Prodrugs and Nanomedicine
Theranostics, a concept that combines therapeutics and diagnostics, has the vast potential to play a significant role in disease treatment. Research in the Sui Lab focuses on developing advanced theranostic prodrugs and nanomedicine, specifically engineered drug-containing compounds and nanoparticle-based drug delivery systems that integrate therapy and diagnosis into a single molecular system, with targeted delivery and stimulus-responsive activation. They remain inactive (non-toxic) in the bloodstream and organ tissues until activated by a disease-specific trigger (e.g., an enzyme, pH, redox state, hypoxia, or external light irradiation), at which point they simultaneously release therapeutic drug molecules and emit a detectable signal (e.g., fluorescence or MRI contrast), achieving spatiotemporal precision in disease treatment, minimized systemic toxicity, real-time monitoring of biodistribution and treatment response, and optimized treatment compliance and personalization.
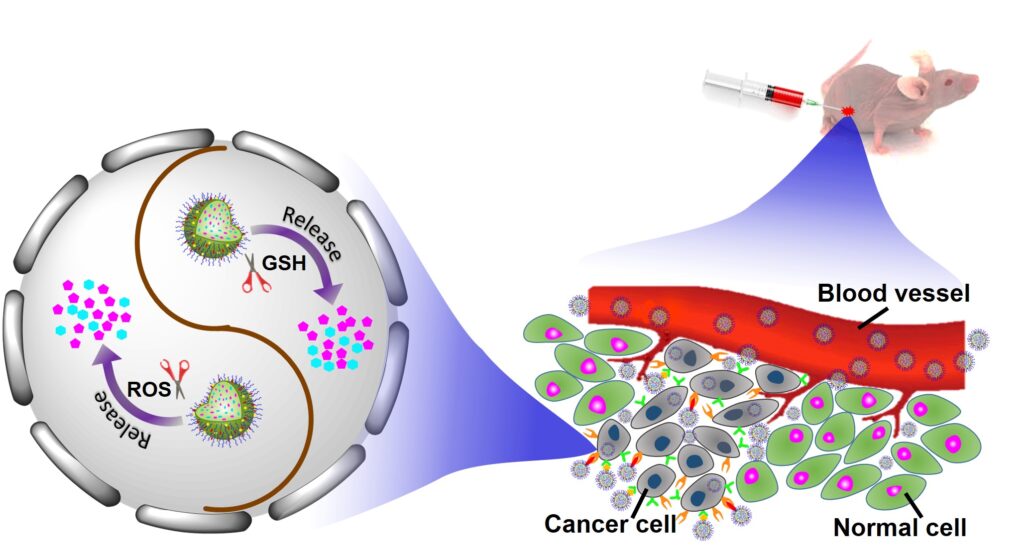
2. Biodegradable Smart Polymers and Gel Materials
Biodegradable smart polymers and gel materials have emerged as a versatile and dynamic class of biomaterials with immense potential in biomedical applications. These materials are engineered to exhibit environment-responsive behavior, such as changes in pH, temperature, redox state, or enzymatic activity, while simultaneously maintaining biocompatibility and biodegradability, two critical requirements for clinical use. By integrating stimuli-responsive features with tailored degradation profiles, these smart materials offer spatiotemporal control over drug release, tissue interaction, and therapeutic functionality. One of the most compelling attributes of biodegradable smart polymers and hydrogels is their ability to mimic the extracellular matrix (ECM), thereby providing a supportive environment for cell adhesion, proliferation, and tissue regeneration. Their soft, hydrated networks make them ideal candidates for applications in controlled drug delivery, tissue engineering, wound healing, and minimally invasive injectable therapies. Furthermore, the degradation of these materials into non-toxic byproducts eliminates the need for surgical removal, thereby reducing patient risk and improving compliance. We strive to develop biodegradable and biocompatible smart polymers and polymeric gels that serve as biosafe and sustainable platforms for regenerative medicine, personalized and responsive therapies, and intelligent biomedical devices.


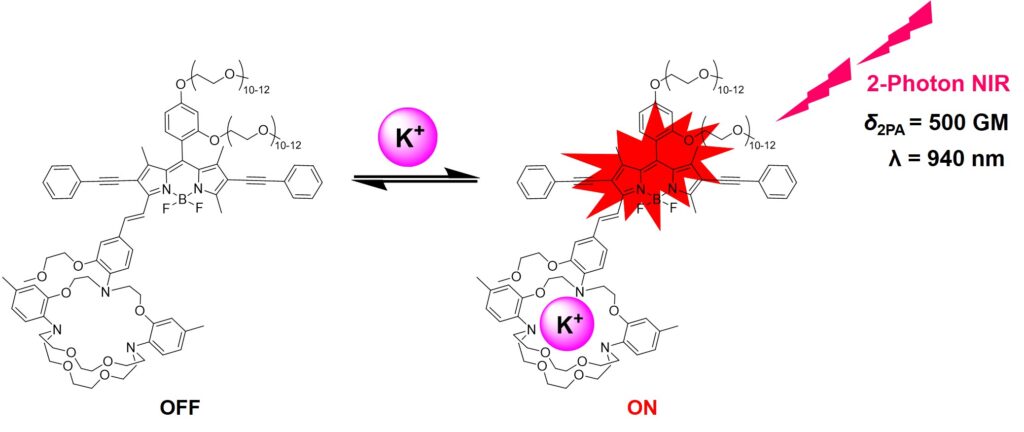
3. NIR Fluorescent Diagnostic Biosensors
Near-infrared (NIR) fluorescent diagnostic biosensors represent a transformative class of molecular tools that integrate high-sensitivity optical detection with biological specificity for disease diagnosis and monitoring, offering significant advantages over traditional visible-light-based systems. Chief among these benefits are their ability to achieve deeper tissue penetration, reduced light scattering, lower phototoxicity, and minimal interference from tissue autofluorescence, which collectively result in superior signal-to-noise ratios during in vivo imaging, making them particularly suitable for real-time, non-invasive imaging of biological targets. In the context of biomedical diagnostics, their compatibility with theranostic platforms further enhances their clinical potential, enabling simultaneous diagnosis and treatment. Our research group is dedicated to developing NIR fluorescent biosensors as powerful tools for early disease detection, monitoring hypoxic conditions, evaluating therapeutic responses, and guiding image-assisted surgeries and medicinal treatments. These biosensors are poised to play an increasingly central role in precision medicine and real-time bioimaging applications.
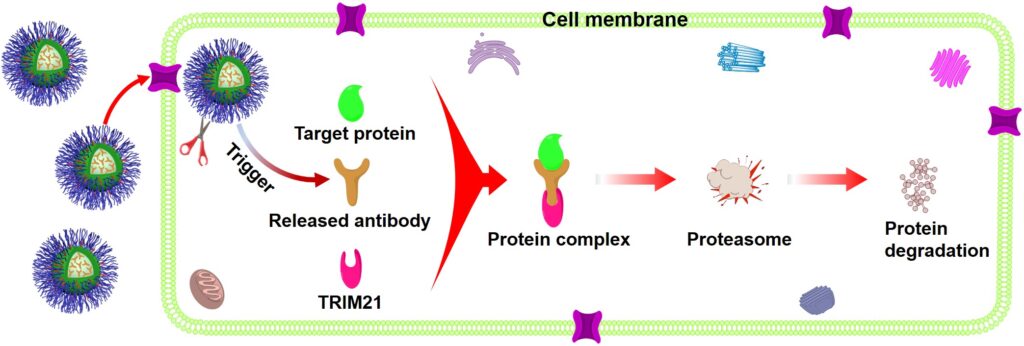
4. Targeted Protein and Gene Therapy
Targeted protein and gene therapy represent cutting-edge approaches in the field of precision medicine, aiming to treat diseases at their molecular root with high specificity and minimal side effects. These therapies are designed to correct or modulate dysfunctional cellular processes by either delivering therapeutic proteins directly or altering gene expression within specific cells or tissues. It is well known that proteins and nucleic acids cannot enter cells on their own due to the impermeability of the cell membrane, which severely hinders the biomedical applications of therapeutic proteins and genome engineering. We make efforts to seek advanced delivery methods for transporting proteins and DNA/RNA into targeted cells by means of agent-loaded nanosystems, in which protein and nucleic acid molecules are covalently conjugated via stimuli-responsive and self-immolating linkers, allowing for the traceless release of the molecules with their native therapeutic effects within cells, which creates a powerful toolkit for addressing diseases at their source, paving the way for more personalized, durable, and effective treatments.
5. Synergistic Chemo-/Photo-/Immuno-/Epigenetic Therapy
Synergistic therapy is an innovative therapeutic strategy that combines two or more treatment modalities to achieve a collective effect greater than the sum of their individual actions via targeting multiple pathways simultaneously. By leveraging complementary mechanisms of action, synergistic therapy aims to enhance treatment efficacy, reduce drug resistance, lower required dosages, and minimize side effects. This strategy is increasingly utilized in the treatment of complex diseases, including cancer, infectious diseases, and neurological disorders. Our research goal is to integrate multiple therapeutic approaches, including chemotherapy, phototherapy (PDT and PTT), immunotherapy, and epigenetic therapy, into a single, well-designed smart nanosystem to establish more powerful therapeutic techniques, thereby overcoming the limitations of monotherapies, such as drug resistance, systemic toxicity, adverse effects, and limited therapeutic windows.